Routine Testing
- Antibody Screen
ABSCN-5 Includes: SRV, SIV, STLV, Herpes B Surrogate Marker, Measles
ABSCN-8 Includes: SRV, SIV, STLV, Herpes B Surrogate Marker, Measles, RhCMV, SFV, RRV
ABSCN-Custom Includes: ABSCN-8 and addtional agents by request (SV40, LCV, custom).
The Antibody Screen panels utilize Multiplex Microbead ImmunoAssays to detect the host's humoral immune response to various infectious agents. The panels simultaneously detect virus-specific antibodies in serum, plasma or other body fluids. The antibody screen assays are run on a Luminex platform using panels of microbeads coated with purified antigens. Each antigen bead has uniquely identifiable spectral signature. Microbeads are incubated with diluted serum samples so that any specific antibodies present will bind to the matching antigen coated beads. The beads are washed, and reacted with phycoerythrin-conjugated detection reagents. After additional washing, independent gated events for each bead are counted and analyzed in a Luminex cytometer. The median fluorescence intensity (MFI) of antigen-coated bead sets is compared to control coated beads in each serum. These assays were designed as screening assays with optimal sensitivity and require confirmatory testing by more specific methods.
The minimum sample requirement is 1 ml of serum or plasma sent frozen.

- WB (Western Blot)
Antibody Screen (ABSCN) results can be confirmed by Western Blot. This method allows visualization of antibody reactivity specific to viral (and non-viral) proteins as separated by molecular weight.
Individual WB's available for: SRV1, SRV2, SRV5, SIV and STLV.
In the WB assay, if specific antibody is present in the serum sample it will bind to the specific antigen bands on the PVDF or Nitrocellulose strip. The presence of antigen-bound antibody is detected by the addition enzyme conjugated detector antibody. Substrate is added that will yield a color change where the enzyme has been bound (positive for antibody).
The minimum sample requirement is 1 ml of serum or plasma sent frozen.
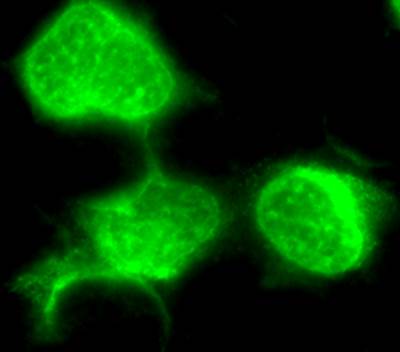
- IFA (Immunofluorescence Assay)
Antibody Screen (ABSCN) results can be confirmed by IFA.
Individual IFA's available for: Measles, SFV, RRV, SV40, LCV and Varicella.
The Immunofluorescence assay (IFA) can be used to detect antibodies to specific virus in infected cells coated on microscope slides. In this assay, test and control serum samples are reacted on the test antigen infected and uninfected control cells. If specific antibody is present in the test sera, it will bind to the test antigen infected and not the uninfected control cells. The specific antibody - test antigen binding is detected by binding a secondary, fluorescent conjugated detector antibody. The signal is observed by fluorescence-microscopy.
The minimum sample requirement is 1 ml of serum or plasma sent frozen.
- PCR (Polymerase Chain Reaction)
Individual PCR available for: SRV (1-5), SFV, STLV, RRV and SIV.
PCR assays are used to detect viral nucleic acids (DNA, RNA, proviral DNA) in various body fluids, whole blood or tissue. This assay allows us to detect and amplify very small quantities of viral or proviral nucleic acid. The appropriate nucleic acid is extracted from blood or tissue and then amplified in the PCR assay. All of our current assays use Taqman1 or Real Time PCR. Probes and primers have been designed to amplify specific viral gene sequences. In addition, probe and primers to amplify the housekeeping gene oncostatin M (OSM) which encodes a cytokine are run in each reaction. The OSM gene is diploid in all primate cells allowing for cell quantification. The OSM signal is used to assure that we obtained amplifiable DNA for all samples. Although for many viruses a PCR result is diagnostic, for SRV, both PCR and serology are necessary to rule out infection.
The minimum sample requirement is 3 ml of heparinized whole blood sent at room temperature to arrive within 24 hours of collection.
- GIFN for TB (Gamma-interferon)
In vitro assays for Interferon Gamma (IFN-g) response to tuberculin antigens represent a refinement of the lymphocyte stimulation assay. As soon as possible following collection, aliquots of heparinized whole blood are stimulated with control and TB antigens in pre-loaded tubes. After 24 hours incubation, the concentration of IFN-g in the supernatant plasma of each aliquot is determined by enzyme immunoassay. In experimental primate infection models, cell mediated immune responses are detectable in vitro 2 to 4 weeks after infection. IFN-g is a critical cytokine in the cell mediated immune response to tuberculin antigens, including the DTH response measured by the TST, and in the host immune response to infections with tuberculous mycobacteria. Primagam (Prionics, USA Inc, La Vista, NE) is a commercial version of this assay using bovine and avian purified protein derivatives (PPD) as antigens.
Please contact us to arrange to receive instructions and pre-loaded stimulation tubes to be filled, incubated and processed on-site immediately following collection.